
Question: I have been reading about "giant molecular clouds" what are they and what are they made of? - Benji, Portland OR
Answer: Giant molecular clouds - such as that shown (Rosette Nebula) consist mainly of molecular hydrogen (H2) but other molecules can also be present including:
OH Hydroxyl radical
CO carbon monoxide
HCN Hydrogen Cyanide
H2O water
NH3 Ammonia
H2 CO Formaldehyde
HC2 H Acetylene
CH4 Methane
HCOOH Formic acid
CH2 OH Methyl alcohol
CH2 CN Methyl cyanide
CH3 NH2 Methylamine
CH2 CH2 CN Ethyl cyanide
HCOOCH3 Methyl formate
This is just to give you an idea of how many different molecules can be found in these molecular clouds, usually associated with a type of nebula containing what we call HI regions (neutral hydrogen) as well as H II regions (single ionized hydrogen) Most of the 'exotic' molecules listed, to be sure, make up only a relatively small proportion of the total mass of such clouds. They were also largely discovered through radio observations beginning in the 1960s.
We acknowledge here that optical observations alone aren't the most practical for detecting such component molecules given different energy states can arise depending on how the atom vibrates or the molecule spins.
Here we can think of the internal energy (U) of a given molecule - say hydrogen H2- and note there are three degrees of freedom or contributions: translational, rotational and vibrational
U = U(transl.) + U(rotational) + U (vibrational)
On average then, the available energy is equally shared by each independent degree of freedom, acknowledging at once that molecules can not only move from point a to point b in space (translation), but also rotate and vibrate. Each of these contributes (½ kT) of energy on average. For the different translational velocity directions, i.e. in 3 space directions x, y, and z (vx, vy and vz ) we than have a total contribution of 3((½ kT) = 3kT/2. To obtain the rotational energy contributions we can use the "dumbbell" model shown in the graphic:
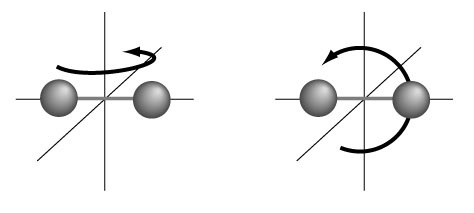
where the system can rotate either in the z-plane (e.g. about the x-axis) or in the x-plane (about the z-axis). Since there is (½ kT) of energy for each of these then the total contributed to internal energy U is: 2((½ kT)= kT. If we finally add in the possible vibrations (visualizing the dumbbell atoms connected via 'springs') we get one more kT contribution to make: 5kT/2 + kT = 7kT/2, so U = 7kT/2.
Having stated the relative abundance of most molecules in giant molecular clouds are small, i.e. relative to hydrogen's, it is also useful to note that the most common of those other molecules are carbon monoxide, ethyl alcohol and water. Again, in order to remain as molecules these need to be localized in dark, denser and colder regions - which, however, often lie close to H II regions. (One of the nearest molecular clouds is situated behind the Orion Nebula)
In terms of density, the typical molecular cloud region has average densities ranging from 108 - 1012 molecules per cubic meter. This compares to a density of 108 molecules per cubic meter for the interstellar medium and about 1,000 atoms per cubic meter near the edge of our solar system. The total mass of these giant ,molecular clouds generally ranges from 104 - 107 solar masses and 10 5 solar masses is typical. Typical low density molecular clouds can extend 30 light years across with a "core" of perhaps 0.5 Ly. These "cores" are generally at a low temperature of 10 K and are where densities can attain 1012 molecules per cubic meter.
Interestingly, giant HII regions, i.e. those surrounding young, massive stars, often connect to giant molecular clouds. In some cases - like the Rosette nebula- models seem to show a "co-moving" HII shell with an HI molecular cloud.
-------------------
Readers who are confident they are up to it can read about this model for the Rosette in a recent paper from the Monthly Notices of the Royal Astronomical Society.
No comments:
Post a Comment